Overview Ultraviolet (UV) Light
Ultraviolet (UV) Light refers to the region of the electromagnetic spectrum that occurs between the visible and x-ray spectrums. Light may be referred to by its wavelength, measured in nanometers (nm). The Ultraviolet wavelength range is specified as 10 nm to 400 nm and is not visible to the human eye, because it has a shorter wavelength and higher frequency than the light our brain perceives as images. Visible light, detectable by the human eye, is between 380nm and 760nm. Red is the light with the longest wavelength, and Violet is the light with the shortest wavelength. Therefore, light with a wavelength longer than any light in the visible spectrum is called Infrared Light, and light with a wavelength immediately shorter than any light in the visible spectrum is called Ultraviolet Light.
There are three types of UV radiation that are classified according to their wavelength. They differ in their biological activity and the extent to which they can penetrate the skin.

Summary of Ultraviolet Light:
- Ultraviolet means "beyond violet" (meaning "beyond" in Latin), and violet being the color of the shortest wavelength of visible light
- Ultraviolet (UV) light is electromagnetic radiation
- UV light has a wavelength that is shorter than that of visible light, but longer than x-ray light and it is invisible to the human eye
- Categorized by wavelength
- UV can be subdivided based on its wavelength, as measured in nanometers. It is typically divided into near UV (200-380nm) and extreme or vacuum UV (10-200nm)
- The sun is the greatest source of UV light
- The sun emits ultraviolet light in both near UV and vacuum UV wavelengths, but because of absorption in the atmosphere's ozone layer, 99% of the ultraviolet light that reaches the Earth's surface is 315-380nm (UV-A)

Ultraviolet Light for Medicine
In medicine, ultraviolet radiation, called UV radiation, comes from special lamps or a laser and is used to treat certain skin conditions such as psoriasis, vitiligo, and skin tumors of cutaneous T-cell lymphoma.
UV-A Or UV-B Light for Therapeutic Phototherapy
Depending on the application, UV Light used for Therapeutic Phototherapy can utilize UV-A or UV-B light.
UV-A & PUV-A Light Spectrum
UV-A is commonly administered with a drug called psoralen, which sensitizes the skin to the UV-A light increasing the effect of treatment. This type of treatment is called PUV-A (the “P” standing for psoralen). PUV-A has a long history of being very effective at treating a variety of skin disorders.
UV-B Light Spectrum
In studies evaluating specific UV-B wavelengths and their therapeutic value versus erythema (reddening of the skin), it was shown that erythema peaked at a UVB light wavelength of 297nm continuing downwards towards 280nm (minimum of UVB range), fell off sharply above 300nm, and was the least nearing 310nm up to 320nm. This means that for the same dosage of UV-B light, much more erythema, (reddening of the skin) are produced in the skin. UV-B is commonly administered by itself but may also be administered in combination with other medications, for example coal tar (e.g. Goeckerman regimen).
UV-B – Narrow Band UV-B (NV – UV-B)
Narrow-Band UVB (NB-UVB) is UV-B light primarily between 300 and 320nm, with a peak near 310nm, and has been shown to be more effective than UV-B, and as effective as PUV-A. These findings prompted the UV light industry to introduce “Narrow-Band” UV-B lamps, or NB-UV-B lamps, which are able to provide light nearing the 310nm peak wavelength that can be delivered in far higher dosages with less side effects than light at 297nm.
Targeted UV Phototherapy
Targeted UV Phototherapy refers to administration of UV light, via an elemental gas lamp, excimer laser or other sources to a small area of symptomatic skin less than 4 cm2. The light administered is typically in the UV-B range (between 280nm and 320nm) though it can be in the UV-A range as well.
Targeted UV phototherapy devices consist of a wand connected to a stationary base console. Due to the small treatment area size of the handheld, many dosages are administered to the symptomatic skin, each dosage lasting several seconds.
Previous treatment options required exposing the patient's entire body to UV radiation, which can have harmful side effects, and limits the possible duration of treatment. Skin affected by psoriasis and other conditions can withstand far greater doses of UV radiation than the non-affected skin, a more targeted approach can clear conditions by using higher doses, reducing total treatment time.
Prior to a targeted UV phototherapy treatment, the Minimal Erythemic Dosage (MED) for each patient is usually determined. Guidelines exist for determining what multiple of the MED will be administered to the patient for treatment. The MED is the amount of UV light that causes reddening of the skin about 8 hours after exposure. Because the UV light is only administered to the affected skin, healthcare providers can administer intense UV dosages, up to several times as much as the patient’s MED. As the therapeutic effects of the UV light on skin presenting with psoriasis, eczema, and vitiligo are more pronounced because more UV light is administered to them, less visits to the healthcare provider’s clinic are required. For example, plaque psoriasis typically clears in as few as 6 to 10 treatments, generally administered twice per week over 3 to 5 weeks.
While non-targeted phototherapy has been in use for decades, targeted phototherapy is a much more recent advancement in the treatment of psoriasis. It can only be administered by a healthcare provider.
Many health insurance providers cover both targeted and non-targeted UV phototherapy. The type of psoriasis and severity, as diagnosed by the patient’s healthcare provider, plays a large role in health insurance reimbursement. The patient should always make certain their healthcare provider is aware of how their Quality of Life is impacted by their disease.
Summary Targeted UV Phototherapy
- Recent advancements for skin treatments
- Releases a smaller range of ultraviolet light
- Affects only unhealthy skin while sparing healthy skin
- Reduced harmful side effects
- Can be applied at higher doses
- Clears psoriasis faster and produce longer remissions
- May require fewer treatments per week
Mechanism of Action Of UV-A/UV-B Phototherapy
UV-A/UV-B phototherapy works by shining UV radiation onto skin, which reduces the immunosuppressant effect of the skin and helps ameliorate conditions including psoriasis, vitiligo, alopecia, eczema, and seborrheic dermatitis. UV phototherapy works through local rather than systemic effects, therefore without the side effect of profound systemic immunosuppression. This action is why it has kept its place in dermatology even in the era of the biological agents.
UV radiation exerts a multitude of biological effects within the skin.
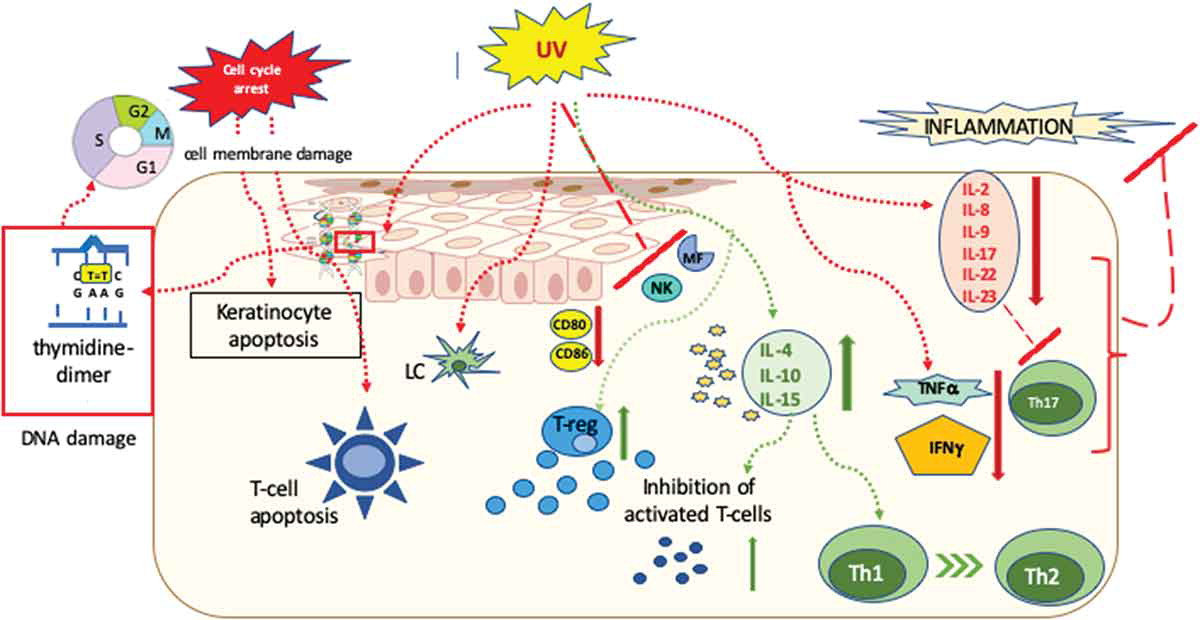
In general, the effects of UV radiation can be divided into two groups: immediate and delayed effects. The immediate effects, such as membrane and DNA damage, induction of cytoplasmatic transcriptional factors and isomerization of chromophores (such as urocanic acid) have been known since 1967, these induce instantly growth arrest and apoptosis.
There is a direct change of molecular structure in DNA due to photon absorption after UV-B which inhibits the DNA transcription machinery and causes cell cycle arrest in human fibroblasts and epidermal cells (phototype I reaction). In the case of photochemotherapy, when the skin is treated with a photosensitizing drug (psoralen) subsequently followed by UV-A radiation, the interaction of photon-excited aromatic molecules with molecular oxygen generates reactive oxygen species, which cause many alterations in the cells, such as DNA damage, cell membrane damage via lipid peroxidation and interaction with signaling pathways (phototype II reaction).
The short-term effects are reversed 48 to 72 hours after irradiation, as the DNA repair starts within an hour after irradiation, and the cells start then to proliferate again. UV radiation has the ability to reverse the skin’s disturbed vessel architecture as well, therefore in psoriatic lesions it normalizes the characteristically elongated capillary loops. Delayed effects of UV radiation include the inhibition of both the adaptive and innate immune cells that results in immunosuppression.
One of the major mechanisms of action of UVB light in the treatment of inflammatory dermatoses is the cytotoxic effect on epidermal T cells, where the mechanism of cell death is apoptosis. Among the diverse effects on inflammatory cells, apoptosis induction in epidermal and dermal T cells seem to be the most important mechanism of action of UV radiation induced immunosuppression. Although keratinocytes are more resistant to UV radiation compared to that of
T cells, UV-B induced apoptosis in keratinocytes also plays a key role in the clearance of psoriatic plaques. Furthermore, UV-B and PUV-A activate
T regulatory (Treg) cells, and stimulation of Tregs results in the downregulation the overreactive immune response in psoriasis.
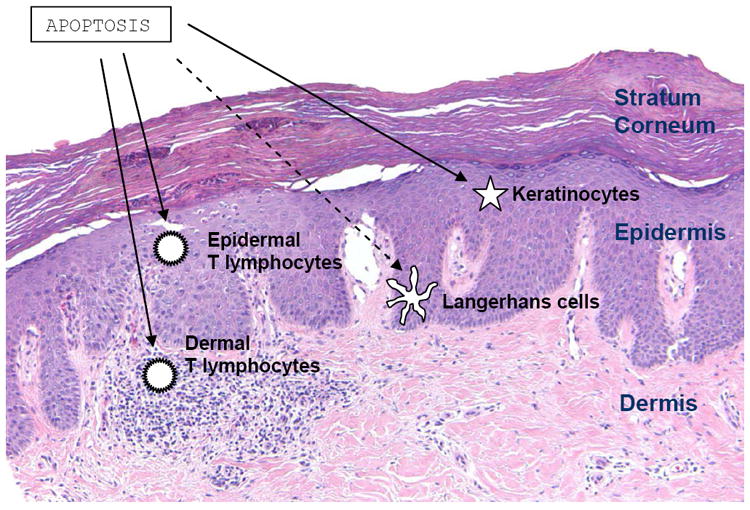
UV radiation affects antigen presenting cells as well. After 7 UV treatment sessions the number of Langerhans cells decreases by 90%, which alters the deviated antigen presentation. Furthermore, the photo-oxidative stress causes cytoskeleton damage in the remaining dendritic cells, which inhibits the further stimulation of T-cells. Cytokine secretion, as well as the number of macrophages, were also diminished by UV radiation. Through the action of the reactive oxygen species UV light switches off the activity of neutrophils and inhibits the NK cell function as well.
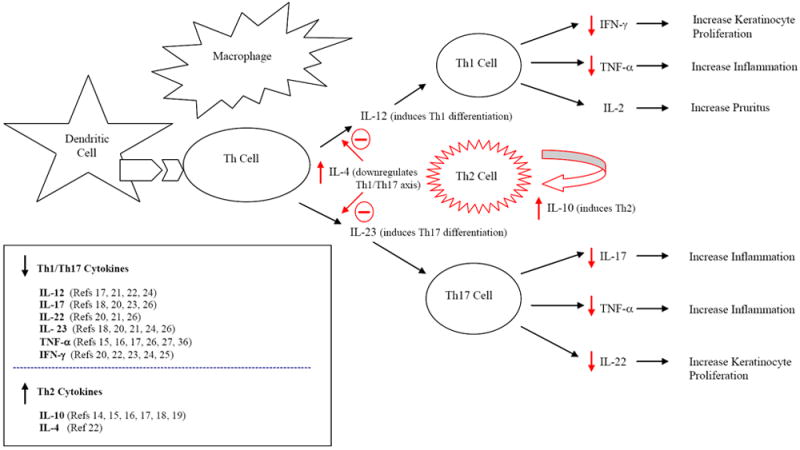
These cellular effects are accompanied by altering the cytokine milieu, the suppression of inflammatory cytokines IL-2, IL-8, IL-9, IL-17, IL-22 and IL-23, TNF-a and IFN-g, and induction of the immunosuppressive cytokine IL-10 also contributes to the cessation of the inflammation.
Summary of The Mechanism of Action and Effects of UV-B:
- UV-B radiation is absorbed by DNA and urocanic acid
- Alteration of cytokine profile
- Induction of apoptosis
- Promotion of immunosuppression
- Alters antigen-presenting cell activity
- In psoriasis, it lowers peripheral natural killer cell activity, lymphocyte proliferation and immunoregulatory cytokine production by Th1 (IL-2, IFN-alpha) and Th2 (IL-10) T-cell populations
- UV-B results in erythema, which begins 2–6 hours after exposure, peaks at 12–18 hours after exposure and generally persists for 48 hours
SUPPORTING DOCUMENTS
Phototherapy in Psoriasis: A Review of Mechanisms of Action
Journal of Cutaneous Medicine and Surgery
SUMMARY:
Phototherapy is one of the most efficacious treatment options for psoriasis. New, emerging studies are beginning to define the biological mechanisms by which phototherapy improves psoriasis. Phototherapy acts through a combination of pathways to confer therapeutic benefits in psoriasis, and these different modalities may help explain its particular usefulness in treating this cutaneous disease.
Link - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736829/
How Do UV Lamps in UV-A & UV-B Phototherapy Work?
- Power is applied to the lamp electrodes
- An electrical arc is generated from ionized gas, or gas mixtures which conduct electricity
- Current is limited from the power source to protect the lamp and supply wiring.
- As the arc temperature rises, mercury in the lamp converts to a gaseous vapor state
- Mercury vapor conducts electricity, completing the circuit.
- Lamp output in the UV range depends on the amount of mercury and vapor pressure of mercury in the lamp
History and Discovery of Ultraviolet
UV radiation was discovered in 1801 when the German physicist Johann Wilhelm Ritter conducted an experiment to investigate the existence of energy beyond the violet end of the visible spectrum. Knowing that photographic paper would turn black more rapidly in blue light than in red light, he exposed the paper to light beyond violet. The paper turned black, proving the existence of ultraviolet light.
He called these invisible rays "(de-)oxidizing rays" (German: de-oxidierende Strahlen) to emphasize chemical reactivity and to distinguish them from "heat rays", discovered the previous year at the other end of the visible spectrum. The simpler term "chemical rays" was adopted soon afterwards, and remained popular throughout the 19th century, although some said that this radiation was entirely different from light (notably John William Draper, who named them "tithonic rays". The terms "chemical rays" and "heat rays" were eventually dropped in favor of ultraviolet and infrared radiation, respectively. In 1878 the sterilizing effect of short-wavelength light by killing bacteria was discovered. By 1903 it was known the most effective wavelengths were around 250 nm. In 1960, the effect of ultraviolet radiation on DNA was established.
Modern phototherapy began with Nobel Prize winner Niels Finsen who developed a “chemical rays” lamp with which he treated patients with skin tuberculosis. However, it took several decades until phototherapy was introduced anew into the dermatological armamentarium. It was the development of photochemotherapy (PUV-A) in 1974 that marked the beginning of a huge upsurge in Photodermatology.
In 1982, PUV-A therapy (the use of the photosensitizing drug Psoralen + UV-A), was approved by the FDA upon completion of one the largest clinical studies in the history of dermatology, a 1380 patient, sixteen center trial led by the Massachusetts General Hospital and fifteen other major clinical centers around the country.
In the early 1990’s, another advance, Narrowband UV-B phototherapy, was introduced. Narrowband UV-B use has since become predominant because it uses a specific, narrow, spectrum of energy that has been clinically shown to be maximally effective.
